Therapeutic Goods Administration (TGA) Compliance Services
The Therapeutic Goods Administration (TGA) regulates medical devices in Australia. Compliance with TGA requirements is essential for manufacturers aiming to sell their medical devices in the Australian market.
What is TGA Compliance?
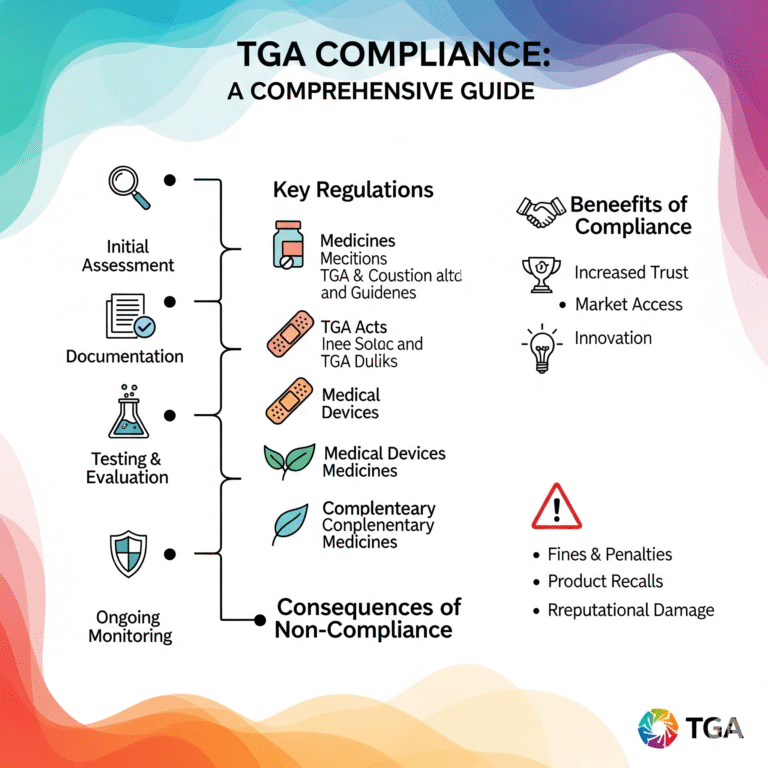
TGA compliance means fulfilling all regulatory requirements defined by the Australian Therapeutic Goods Act and related regulations. Medical devices must be included in the Australian Register of Therapeutic Goods (ARTG) before they can be marketed or supplied in Australia.
Key elements of TGA compliance include:
- Device classification (based on GMDN and risk)
- Conformity assessment procedures
- Technical documentation and evidence of safety & performance
- Appointment of an Australian Sponsor (if overseas manufacturer)
- ARTG inclusion and post-market obligations
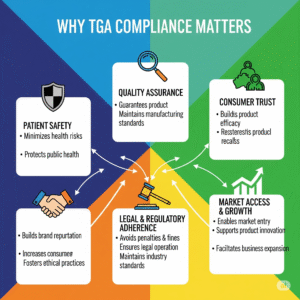
Why is TGA Compliance Important?
TGA compliance is mandatory for legally supplying medical devices in Australia. It:
- Ensures market access across Australia
- Validates the safety and performance of your device
- Reduces legal and regulatory risks
- Enhances transparency and credibility among healthcare professionals
Without registration, your device cannot be legally marketed in the UK.
Our TGA Compliance Services
Lisora offers complete support for meeting TGA requirements effectively and efficiently. Our services include:
- Device classification and regulatory strategy
- Conformity assessment preparation
- Assistance with technical documentation
- Conformity assessment preparation
- Device classification and regulatory strategy

Why Choose Lisora for TGA Compliance?
Compliance Experts
Expertise in TGA regulations and global submission process
Regulatory Alignment
Aligning with TGA and ISO 13485 standards for full success
End-to-End Support
From classification to complete post-market compliance quickly
Timely Execution
Focused on speed, quality, and accuracy for every medical device
Frequently Asked Questions (FAQs)
1. What is TGA and what does it regulate?
The Therapeutic Goods Administration (TGA) is the regulatory authority responsible for the quality, safety, and efficacy of therapeutic goods, including medical devices, in Australia.
2. What is ARTG inclusion?
Before marketing a medical device in Australia, it must be included in the Australian Register of Therapeutic Goods (ARTG).
3. Who can apply for ARTG inclusion?
Only an Australian-based sponsor can apply for ARTG inclusion. Overseas manufacturers must appoint a sponsor in Australia.
4. What documentation is required for TGA compliance?
Manufacturers must provide technical documentation, risk analysis, conformity assessment evidence, and post-market surveillance plans.
5. Is TGA compliance aligned with ISO 13485?
Yes. TGA accepts ISO 13485 certification as part of conformity assessment, but may still require additional documentation.
